Determination of Cyanide via Amperometric Detection
Free / Total / Weak Acid Dissociable
Introduction & Principle
Cyanide analysis has long been notorious for the use of volatile, toxic reagents (pyridine, Chloramine T) in the traditional colorimetric assay. About 25 years ago, an alternative technology was developed, based on amperometric detection, which utilizes dilute acid and base solutions as the only reagents.[1] The lack of hazardous reagents and the ease of use led to the new method gaining popularity and finally being promulgated into official USEPA, ISO, and ASTM methods. After receiving official status, amperometric CN methods have quickly become the preferred method for cyanide determination among environmental testing laboratories, mining companies, and industrial operations.
Cyanide in sample solution is made volatile by in-line mixing with hydrochloric or sulfuric acid. The resulting solution is then directed to a gas diffusion cell where it comes in contact with a gas-permeable membrane. Volatile cyanide migrates through the membrane into an acceptor solution of sodium hydroxide. The cyanide-containing acceptor solution is directed into an amperometric detection cell where cyanide concentration is measured as an electric current that cyanide generates when it contacts a polarized silver electrode. The use of gas diffusion greatly improves method robustness by minimizing detrimental effects from the sample matrix. As only gaseous components can pass through the membrane, many potential fouling agents in the sample matrix are completely excluded from the detector.
For total cyanide measurements, an in-line digestion module can automate the process of releasing cyanide from complexes with zinc, copper, cadmium, mercury, nickel, silver, and iron. When the sample passes through the digestion module, it is subjected to UV radiation that breaks down the above metal-cyanide complexes, resulting in liberated cyanide.
Figure 1: instrument setup for total cyanide with autosampler, FIAlyzer-1000 with amperometric detector, and in-line digestion module(L to R)
Application Benefits:
Robustness
Simplicity
Reduced operator workload
Operator safety and environmental consciousness
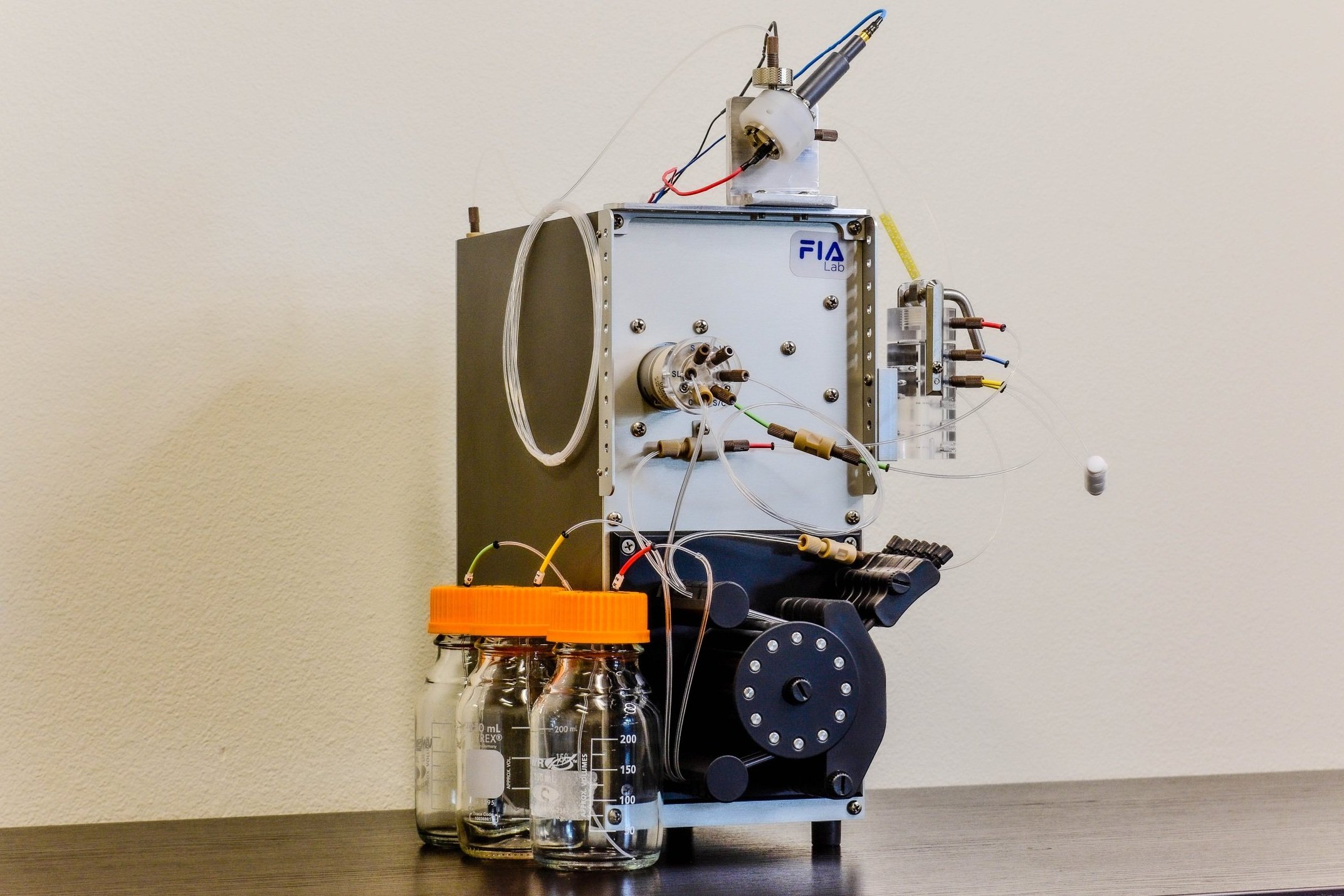
Figure 2: instrument setup with FIAlyzer-1000 with amperometric detector for Free and WAD cyanide
Experimental
Experiments were carried out using the FIAlyzer-1000 Flow Injection Analyzer equipped with an amperometric detector.
The instrument and the related methods are compliant with:
Available cyanide determination by OIA-1677-09 (EPA), 4500-CN-I (Standard Methods), 14403 (ISO) or 6888-09 (ASTM). [CN-W-4-1]
Free cyanide determination by 7237-15a (ASTM). [CN-W-4-3]
Total cyanide (fully automated) determination by D7511−12 (ASTM). This setup would require the addition of an in-line digestion module. [CN-W-4-2]
Total cyanide (semi-automated) determination in post-distillation samples by D7284 (ASTM). In this case, samples are first processed by manual batch distillation. [CN-W-4-1]
Figure 3: Method Schematic for Amperometric Cyanide method (Free/WAD/off-line Total)
Instrument Parameters
Reagent Composition
Carrier (C): Water
Reagent 1 (R1): Acid (WAD or Total) / Buffer (Free)
Reagent 2 (R2): 0.1M NaOH
Sample Volume
417 µL
Detector Setup:
Working Electrode (WE): Ag
Reference Electrode (RE): Ag/AgCl
Counter Electrode (CE): Stainless Steel
WE potential: 0.0 V (vs. Ag/AgCl reference electrode)
Results
Figure 4: Example data of cyanide calibration peaks
Figure 5: Example calibration profile of cyanide range
| Method Performance (µg/L CN) | WAD/Free | Total |
|---|---|---|
| Detection Limit | 0.5 | 1 |
| Reporting Limit | 1 | 3 |
| Range Upper Limit | 500 | 500 |
| Sample Throughput/hr | 40 | 20 |
* If desired, the range can be extended to higher concentrations by decreasing the injected sample volume.
Conclusions
The amperometric cyanide assay is a sensitive, selective and robust method for measuring cyanide in water samples. Robustness stems from incorporating a gas diffusion unit to isolate the amperometric detector from potential interfering components in the sample solution.
References
[1] Milosavljevic E. et al. “Rapid distillationless “free cyanide” determination by a flow injection ligand exchange method”, Environ. Sci. Technol. 29, 1995, p. 426-430.